What Is The Trend In The Size Of The Band Gap As You Move Down The Column Of The Group 4a Elements?
18.6: Group 4A Elements
- Page ID
- 60398
Learning Objectives
- To understand the trends in backdrop and reactivity of the group xiv elements.
The elements of group 14 show a greater range of chemical behavior than any other family in the periodic table. Three of the five elements—carbon, tin, and pb—have been known since ancient times. For example, some of the oldest known writings are Egyptian hieroglyphics written on papyrus with ink fabricated from lampblack, a finely divided carbon soot produced by the incomplete combustion of hydrocarbons (Figure \(\PageIndex{1}\)). Activated carbon is an even more than finely divided form of carbon that is produced from the thermal decomposition of organic materials, such every bit sawdust. Because it adsorbs many organic and sulfur-containing compounds, activated carbon is used to decolorize foods, such every bit sugar, and to purify gases and wastewater.
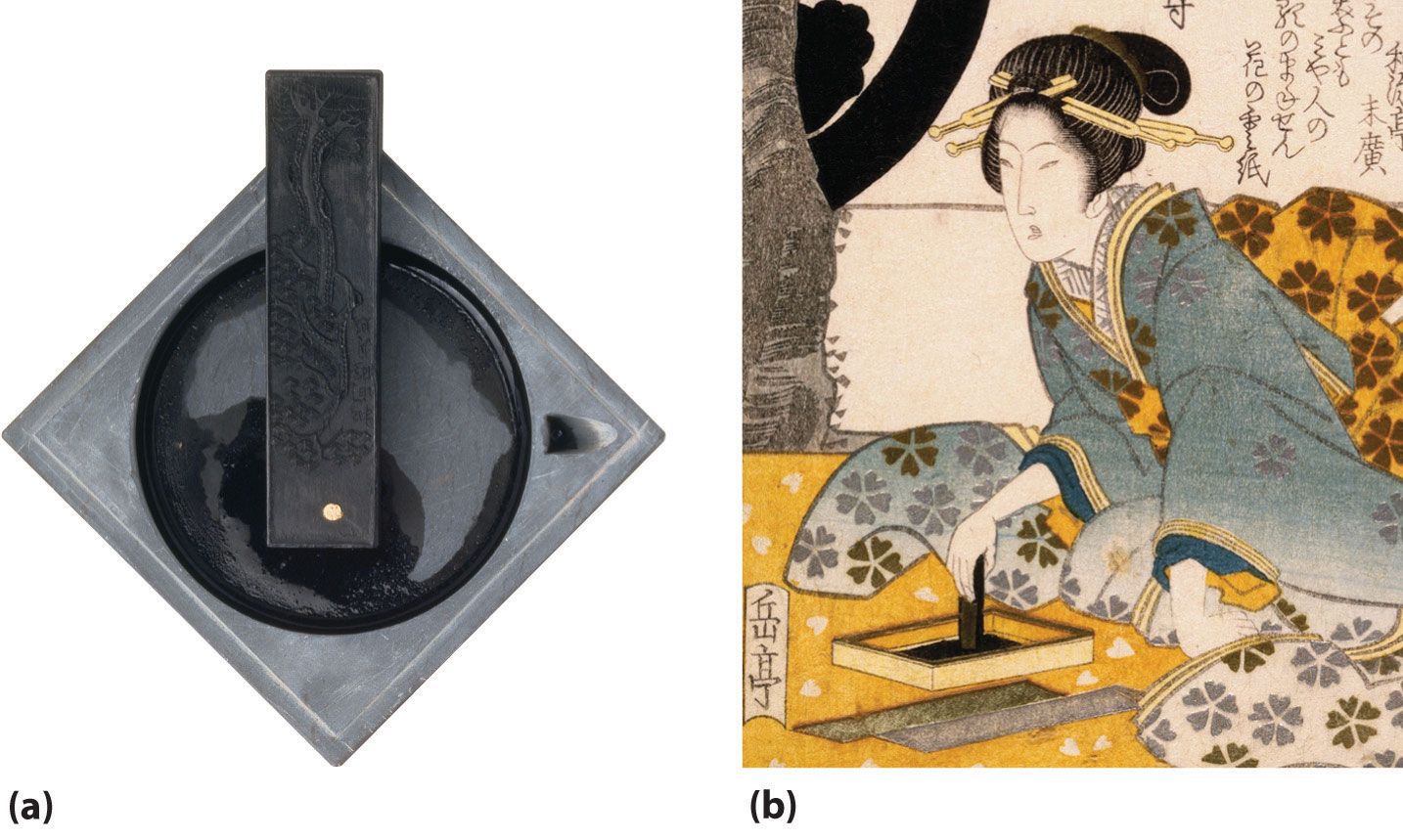
Tin can and lead oxides and sulfides are easily reduced to the metal by heating with charcoal, a discovery that must have occurred past accident when prehistoric humans used rocks containing their ores for a cooking burn. However, because tin and copper ores are often found together in nature, their alloy—statuary—was probably discovered before either element, a discovery that led to the Bronze Age. The heaviest element in group xiv, lead, is such a soft and malleable metal that the ancient Romans used thin lead foils as writing tablets, as well as atomic number 82 cookware and lead pipes for plumbing. (Recall that the atomic symbols for can and lead come from their Latin names: Sn for stannum and Pb for plumbum.)
Although the first glasses were prepared from silica (silicon oxide, SiOii) around 1500 BC, elemental silicon was not prepared until 1824 because of its high analogousness for oxygen. Jöns Jakob Berzelius was finally able to obtain baggy silicon by reducing NaiiSiFsix with molten potassium. The crystalline element, which has a shiny bluish-grayness luster, was not isolated until 30 yr subsequently. The final fellow member of the grouping 14 elements to be discovered was germanium, which was establish in 1886 in a newly discovered silver-colored ore by the German chemist Clemens Winkler, who named the element in accolade of his native country.
Preparation and Full general Properties of the Grouping 14 Elements
The natural abundance of the group 14 elements varies tremendously. Elemental carbon, for example, ranks only 17th on the list of constituents of World'southward crust. Pure graphite is obtained by reacting coke, an baggy form of carbon used as a reductant in the production of steel, with silica to give silicon carbide (SiC). This is then thermally decomposed at very high temperatures (2700°C) to give graphite:
\[\mathrm{SiO_2(s)}+\mathrm{3C(due south)}\xrightarrow{\Delta}\mathrm{SiC(s)}+\mathrm{2CO(g)} \label{\(\PageIndex{ane}\)}\]
\[\mathrm{SiC(southward)}\xrightarrow{\Delta}\mathrm{Si(s)}+\mathrm{C(graphite)} \characterization{\(\PageIndex{ii}\)}\]
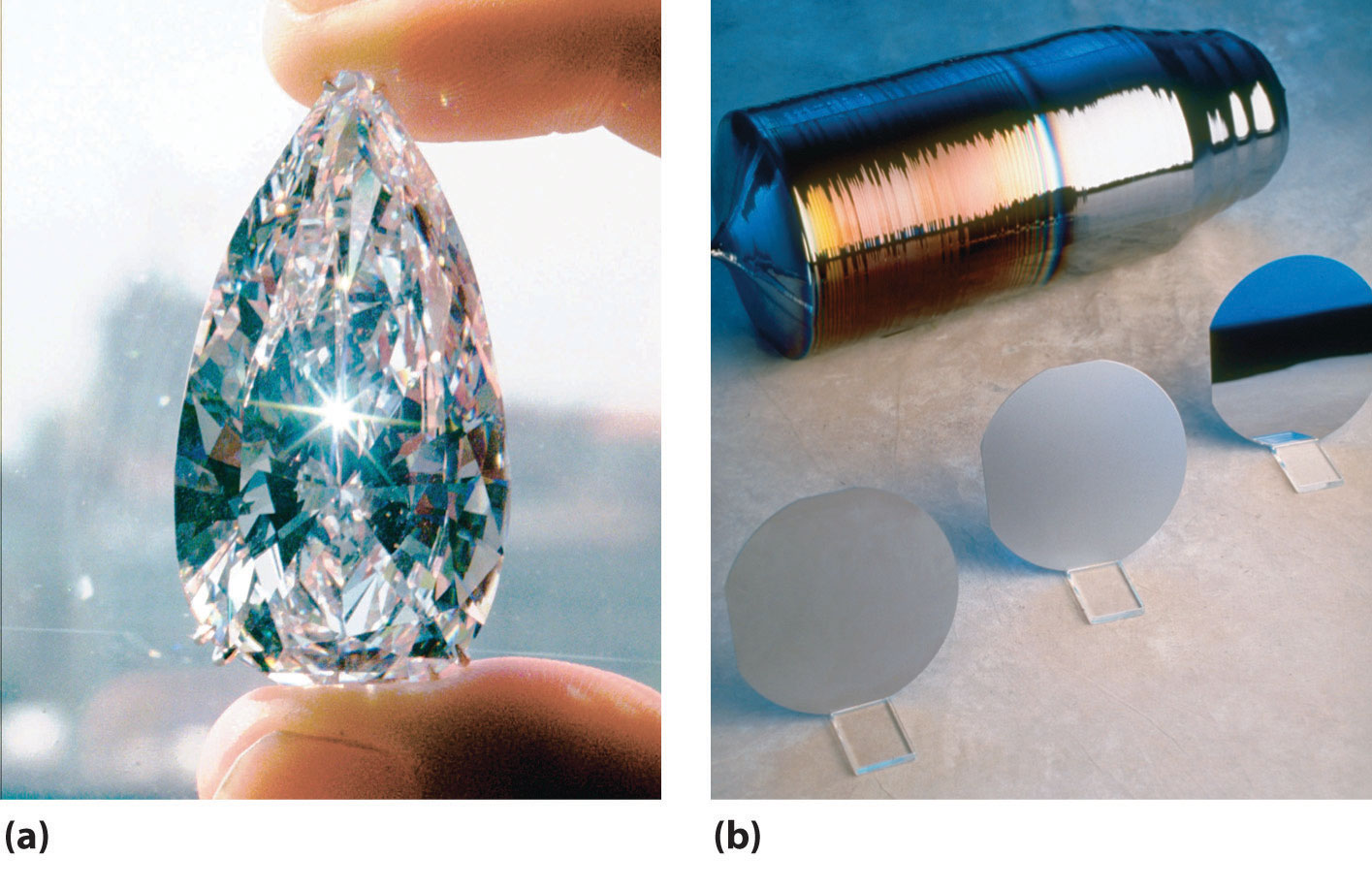
One allotrope of carbon, diamond, is metastable under normal conditions, with a ΔG°f of ii.ix kJ/mol versus graphite. At pressures greater than 50,000 atm, however, the diamond structure is favored and is the most stable form of carbon. Because the construction of diamond is more compact than that of graphite, its density is significantly higher (3.51 grand/cm3 versus 2.2 g/cm3). Considering of its high thermal conductivity, diamond pulverization is used to transfer heat in electronic devices.
The well-nigh mutual sources of diamonds on Globe are aboriginal volcanic pipes that contain a rock called kimberlite, a lava that solidified rapidly from deep inside the Earth. Well-nigh kimberlite formations, however, are much newer than the diamonds they comprise. In fact, the relative amounts of different carbon isotopes in diamond prove that diamond is a chemical and geological "fossil" older than our solar system, which means that diamonds on Earth predate the existence of our sun. Thus diamonds were virtually likely created deep within Globe from primordial grains of graphite nowadays when World was formed (role (a) in Figure \(\PageIndex{2}\)). Gem-quality diamonds tin now exist produced synthetically and have chemical, optical, and concrete characteristics identical to those of the highest-form natural diamonds.
Although oxygen is the well-nigh abundant chemical element on Earth, the next nigh arable is silicon, the next member of group xiv. Pure silicon is obtained past reacting impure silicon with Cl2 to requite SiClfour, followed by the partial distillation of the impure SiClfour and reduction with H2:
\[\mathrm{SiCl_4(l)}+\mathrm{2H_2(thousand)}\xrightarrow{\Delta}\mathrm{Si(s)}+\mathrm{4HCl(g)} \label{\(\PageIndex{3}\)}\]
Several meg tons of silicon are annually produced with this method. Amorphous silicon containing residual amounts of hydrogen is used in photovoltaic devices that convert light to electricity, and silicon-based solar cells are used to power pocket calculators, boats, and highway signs, where admission to electricity by conventional methods is difficult or expensive. Ultrapure silicon and germanium form the basis of the modern electronics industry (part (b) in Figure \(\PageIndex{2}\)).
In contrast to silicon, the concentrations of germanium and tin in World's crust are only i–2 ppm. The concentration of lead, which is the finish product of the nuclear decay of many radionuclides, is 13 ppm, making atomic number 82 past far the nearly abundant of the heavy grouping 14 elements. No full-bodied ores of germanium are known; similar indium, germanium is generally recovered from flue dusts obtained by processing the ores of metals such as zinc. Because germanium is essentially transparent to infrared radiations, it is used in optical devices.
Tin and lead are soft metals that are too weak for structural applications, but because can is flexible, corrosion resistant, and nontoxic, information technology is used as a blanket in food packaging. A "tin tin can," for example, is actually a steel tin whose interior is coated with a thin layer (1–2 µm) of metal tin. Can is also used in superconducting magnets and low-melting-betoken alloys such as solder and pewter. Pure lead is obtained by heating galena (PbS) in air and reducing the oxide (PbO) to the metal with carbon, followed by electrolytic deposition to increase the purity:
\[\mathrm{PbS(southward)}+\frac{3}{2}\mathrm{O_2(thou)}\xrightarrow{\Delta}\mathrm{PbO(south)}+\mathrm{SO_2(g)} \label{\(\PageIndex{iv}\)}\]
\[\mathrm{PbO(s)}+\mathrm{C(south)}\xrightarrow{\Delta}\mathrm{Pb(50)}+\mathrm{CO(g)} \label{\(\PageIndex{5}\)}\]
or
\[\mathrm{PbO(s)}+\mathrm{CO(yard)}\xrightarrow{\Delta}\mathrm{Pb(l)}+\mathrm{CO_2(one thousand)} \label{\(\PageIndex{6}\)}\]
By far the single largest employ of lead is in lead storage batteries. The grouping 14 elements all have ns2nptwo valence electron configurations. All class compounds in which they formally lose either the ii np and the two ns valence electrons or just the two np valence electrons, giving a +4 or +2 oxidation state, respectively. Because covalent bonds decrease in forcefulness with increasing atomic size and the ionization energies for the heavier elements of the group are higher than expected due to a higher Zeff, the relative stability of the +2 oxidation state increases smoothly from carbon to pb.
The relative stability of the +2 oxidation state increases, and the tendency to course catenated compounds decreases, from carbon to atomic number 82 in grouping xiv.
Recall that many carbon compounds contain multiple bonds formed past π overlap of singly occupied 2p orbitals on next atoms. Compounds of silicon, germanium, tin, and pb with the aforementioned stoichiometry as those of carbon, yet, tend to have dissimilar structures and properties. For case, COii is a gas that contains discrete O=C=O molecules, whereas the nearly common form of SiOtwo is the high-melting solid known equally quartz, the major component of sand. Instead of discrete SiO2 molecules, quartz contains a three-dimensional network of silicon atoms that is like to the construction of diamond simply with an oxygen atom inserted between each pair of silicon atoms. Thus each silicon atom is linked to 4 other silicon atoms past bridging oxygen atoms.
The trend to catenate—to grade chains of like atoms—decreases apace as we go downward group 14 because bond energies for both the E–E and East–H bonds decrease with increasing atomic number (where East is any group 14 chemical element). Consequently, inserting a CHii grouping into a linear hydrocarbon such equally n-hexane is exergonic (ΔG° = −45 kJ/mol), whereas inserting an SiH2 grouping into the silicon counterpart of due north-hexane (Si6H14) actually costs free energy (ΔG° ≈ +25 kJ/mol). As a result of this trend, the thermal stability of catenated compounds decreases speedily from carbon to lead.
In Table \(\PageIndex{1}\) "Selected Properties of the Group 14 Elements" we come across, in one case once again, that there is a large difference between the lightest element (C) and the others in size, ionization energy, and electronegativity. As in grouping 13, the 2nd and 3rd elements (Si and Ge) are similar, and at that place is a reversal in the trends for some properties, such as ionization energy, between the fourth and fifth elements (Sn and Pb). As for grouping thirteen, these effects tin be explained by the presence of filled (northward − 1)d and (n − 2)f subshells, whose electrons are relatively poor at screening the outermost electrons from the higher nuclear accuse.
| Property | Carbon | Silicon | Germanium | Tin can | Atomic number 82 |
|---|---|---|---|---|---|
| *The configuration shown does not include filled d and f subshells. | |||||
| †The values cited are for six-coordinate +iv ions in the most common oxidation state, except for C4 + and Siiv +, for which values for the four-coordinate ion are estimated. | |||||
| ‡X is Cl, Br, or I. Reaction with F2 gives the tetrafluorides (EF4) for all grouping 14 elements, where E represents any group xiv chemical element. | |||||
| atomic symbol | C | Si | Ge | Sn | Lead |
| atomic number | 6 | 14 | 32 | fifty | 82 |
| diminutive mass (amu) | 12.01 | 28.09 | 72.64 | 118.71 | 207.two |
| valence electron configuration* | 2sii2p2 | 3s23p2 | 4s24p2 | 5sii5p2 | 6s26p2 |
| melting point/humid point (°C) | 4489 (at 10.3 MPa)/3825 | 1414/3265 | 939/2833 | 232/2602 | 327/1749 |
| density (g/cm3) at 25°C | 2.ii (graphite), 3.51 (diamond) | 2.33 | 5.32 | 7.27(white) | 11.30 |
| diminutive radius (pm) | 77 (diamond) | 111 | 125 | 145 | 154 |
| get-go ionization energy (kJ/mol) | 1087 | 787 | 762 | 709 | 716 |
| most mutual oxidation state | +four | +4 | +four | +4 | +4 |
| ionic radius (pm)† | ≈29 | ≈xl | 53 | 69 | 77.v |
| electron affinity (kJ/mol) | −122 | −134 | −119 | −107 | −35 |
| electronegativity | two.6 | 1.9 | two.0 | 2.0 | 1.8 |
| standard reduction potential (Due east°, V) (for EO2 → E in acidic solution) | 0.21 | −0.86 | −0.eighteen | −0.12 | 0.79 |
| product of reaction with O2 | COii, CO | SiO2 | GeO2 | SnO2 | PbO |
| type of oxide | acidic (CO2) | acidic neutral (CO) | amphoteric | amphoteric | amphoteric |
| product of reaction with N2 | none | Si3N4 | none | Sn3Northiv | none |
| product of reaction with X2 ‡ | CX4 | Half-dozen4 | GeX4 | SnXiv | PbX2 |
| production of reaction with Hii | CHiv | none | none | none | none |
The grouping 14 elements follow the same pattern equally the grouping 13 elements in their periodic properties.
Reactions and Compounds of Carbon
Carbon is the building block of all organic compounds, including biomolecules, fuels, pharmaceuticals, and plastics, whereas inorganic compounds of carbon include metal carbonates, which are institute in substances as diverse equally fertilizers and antacid tablets, halides, oxides, carbides, and carboranes. Like boron in group thirteen, the chemistry of carbon differs sufficiently from that of its heavier congeners to merit a separate word.
The structures of the allotropes of carbon—diamond, graphite, fullerenes, and nanotubes—are distinct, simply they all contain simple electron-pair bonds (Figure vii.xviii). Although information technology was originally believed that fullerenes were a new form of carbon that could be prepared but in the laboratory, fullerenes have been found in certain types of meteorites. Another possible allotrope of carbon has besides been detected in touch fragments of a carbon-rich meteorite; it appears to consist of long chains of carbon atoms linked by alternating single and triple bonds, (–C≡C–C≡C–)due north. Carbon nanotubes ("buckytubes") are existence studied as potential building blocks for ultramicroscale detectors and molecular computers and as tethers for space stations. They are currently used in electronic devices, such every bit the electrically conducting tips of miniature electron guns for flat-panel displays in portable computers.
Although all the carbon tetrahalides (CX4) are known, they are more often than not not obtained by the direct reaction of carbon with the elemental halogens (X2) merely by indirect methods such as the post-obit reaction, where Ten is Cl or Br:
\[CH_{iv(thousand)} + 4X_{2(thou)} \rightarrow CX_{4(fifty,southward)} + 4HX_{(grand)} \label{\(\PageIndex{seven}\)}\]
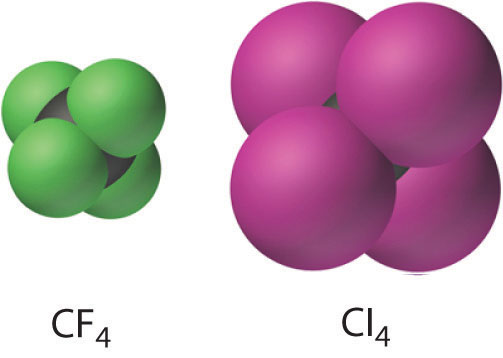
The carbon tetrahalides all have the tetrahedral geometry predicted by the valence-vanquish electron-pair repulsion (VSEPR) model, equally shown for CClfour and CI4. Their stability decreases rapidly equally the halogen increases in size because of poor orbital overlap and increased crowding. Because the C–F bond is virtually 25% stronger than a C–H bond, fluorocarbons are thermally and chemically more stable than the corresponding hydrocarbons, while having a like hydrophobic character. A polymer of tetrafluoroethylene (F2C=CF2), analogous to polyethylene, is the nonstick Teflon lining found on many cooking pans, and like compounds are used to brand fabrics stain resistant (such as Scotch-Gard) or waterproof but breathable (such equally Gore-Tex).
The stability of the carbon tetrahalides decreases with increasing size of the halogen due to increasingly poor orbital overlap and crowding.
Carbon reacts with oxygen to form either CO or COtwo, depending on the stoichiometry. Carbon monoxide is a colorless, odorless, and poisonous gas that reacts with the iron in hemoglobin to form an Fe–CO unit, which prevents hemoglobin from binding, transporting, and releasing oxygen in the blood (see Figure 23.26). In the laboratory, carbon monoxide can be prepared on a small scale past dehydrating formic acid with concentrated sulfuric acid:
\[\mathrm{HCO_2H(l)}\xrightarrow{\mathrm{H_2SO_4(l)}}\mathrm{CO(g)}+\mathrm{H_3O^+(aq)}+\mathrm{HSO^-_4}\label{\(\PageIndex{8}\)}\]
Carbon monoxide as well reacts with the halogens to course the oxohalides (COXii). Probably the best known of these is phosgene (Cl2C=O), which is highly poisonous and was used as a chemical weapon during World State of war I:
\[\mathrm{CO(g)}+\mathrm{Cl_2(g)}\xrightarrow{\Delta}\textrm{Cl}_2\textrm{C=O(g)}\label{\(\PageIndex{9}\)}\]
Despite its toxicity, phosgene is an important industrial chemical that is prepared on a large scale, primarily in the manufacture of polyurethanes.
Carbon dioxide can be prepared on a small scale by reacting almost whatever metal carbonate or bicarbonate table salt with a potent acid. As is typical of a nonmetal oxide, COii reacts with water to grade acidic solutions containing carbonic acrid (H2CO3). In contrast to its reactions with oxygen, reacting carbon with sulfur at high temperatures produces only carbon disulfide (CS2):
\[\mathrm{C(s)}+\mathrm{2S(g)}\xrightarrow{\Delta}\mathrm{CS_2(g)}\label{\(\PageIndex{1}\)0}\]
The selenium analog CSeii is also known. Both have the linear construction predicted past the VSEPR model, and both are vile smelling (and in the case of CSe2, highly toxic), volatile liquids. The sulfur and selenium analogues of carbon monoxide, CS and CSe, are unstable because the C≡Y bonds (Y is S or Se) are much weaker than the C≡O bail due to poorer π orbital overlap.
\(\pi\) bonds between carbon and the heavier chalcogenides are weak due to poor orbital overlap.
Binary compounds of carbon with less electronegative elements are chosen carbides. The chemical and physical properties of carbides depend strongly on the identity of the second element, resulting in three full general classes: ionic carbides, interstitial carbides, and covalent carbides. The reaction of carbon at loftier temperatures with electropositive metals such as those of groups one and ii and aluminum produces ionic carbides, which contain discrete metal cations and carbon anions. The identity of the anions depends on the size of the 2nd element. For example, smaller elements such as beryllium and aluminum give methides such as Exist2C and Al4C3, which formally comprise the Civ− ion derived from methane (CH4) by losing all four H atoms every bit protons. In contrast, larger metals such as sodium and calcium requite carbides with stoichiometries of Na2Cii and CaC2. Because these carbides comprise the C4− ion, which is derived from acetylene (HC≡CH) past losing both H atoms as protons, they are more properly called acetylides. Reacting ionic carbides with dilute aqueous acrid results in protonation of the anions to give the parent hydrocarbons: CH4 or CtwoH2. For many years, miners' lamps used the reaction of calcium carbide with water to produce a steady supply of acetylene, which was ignited to provide a portable lantern.

The reaction of carbon with virtually transition metals at high temperatures produces interstitial carbides. Due to the less electropositive nature of the transition metals, these carbides contain covalent metallic–carbon interactions, which result in different properties: most interstitial carbides are expert conductors of electricity, take high melting points, and are among the hardest substances known. Interstitial carbides showroom a variety of nominal compositions, and they are often nonstoichiometric compounds whose carbon content can vary over a wide range. Among the nigh of import are tungsten carbide (WC), which is used industrially in loftier-speed cutting tools, and cementite (Fe3C), which is a major component of steel.
Elements with an electronegativity similar to that of carbon form covalent carbides, such as silicon carbide (SiC; Equation \(\ref{Eq1}\)) and boron carbide (B4C). These substances are extremely hard, have high melting points, and are chemically inert. For case, silicon carbide is highly resistant to chemical assail at temperatures equally high equally 1600°C. Considering it as well maintains its strength at high temperatures, silicon carbide is used in heating elements for electric furnaces and in variable-temperature resistors.
Carbides formed from group i and 2 elements are ionic. Transition metals form interstitial carbides with covalent metal–carbon interactions, and covalent carbides are chemically inert.
Instance \(\PageIndex{1}\)
For each reaction, explain why the given product forms.
- CO(grand) + Cl2(g) → CliiC=O(g)
- CO(g) + BF3(1000) → FiiiB:C≡O(g)
- Sr(s) + 2C(due south) \(\xrightarrow{\Delta}\) SrCii(s)
Given: balanced chemical equations
Asked for: why the given products form
Strategy:
Classify the type of reaction. Using periodic trends in atomic properties, thermodynamics, and kinetics, explain why the observed reaction products form.
Solution:
- Because the carbon in CO is in an intermediate oxidation state (+2), CO can be either a reductant or an oxidant; information technology is also a Lewis base. The other reactant (Cl2) is an oxidant, then we expect a redox reaction to occur in which the carbon of CO is further oxidized. Because Cltwo is a two-electron oxidant and the carbon atom of CO can be oxidized by 2 electrons to the +4 oxidation country, the product is phosgene (CliiC=O).
- Unlike Cl2, BF3 is not a proficient oxidant, fifty-fifty though it contains boron in its highest oxidation land (+3). Nor can BF3 behave like a reductant. Like whatsoever other species with only six valence electrons, however, it is certainly a Lewis acid. Hence an acid–base reaction is the nigh likely alternative, especially because we know that CO can employ the alone pair of electrons on carbon to act as a Lewis base. The well-nigh probable reaction is therefore the formation of a Lewis acid–base adduct.
- Typically, both reactants behave like reductants. Unless one of them can also behave like an oxidant, no reaction volition occur. We know that Sr is an active metal because information technology lies far to the left in the periodic table and that it is more electropositive than carbon. Carbon is a nonmetal with a significantly higher electronegativity; it is therefore more than likely to take electrons in a redox reaction. We conclude, therefore, that Sr will be oxidized, and C volition be reduced. Carbon forms ionic carbides with agile metals, then the reaction will produce a species formally containing either C4− or C2 2−. Those that contain Civ− unremarkably involve modest, highly charged metal ions, so Sr2 + will produce the acetylide (SrC2) instead.
Exercise \(\PageIndex{1}\)
Predict the products of the reactions and write a counterbalanced chemical equation for each reaction.
- C(s) + excess O2(yard) \(\xrightarrow{\Delta}\)
- C(s) + HiiO(l) →
- NaHCO3(due south) + H2Theniv(aq) →
Answer
- C(s) + excess Otwo(1000) \(\xrightarrow{\Delta}\) CO2(g)
- C(south) + HiiO(50) → no reaction
- NaHCO3(s) + H2Then4(aq) → CO2(yard) + NaHSO4(aq) + H2O(l)
Reactions and Compounds of the Heavier Grouping 14 Elements
Although silicon, germanium, tin, and lead in their +4 oxidation states ofttimes class binary compounds with the aforementioned stoichiometry as carbon, the structures and properties of these compounds are usually significantly different from those of the carbon analogues. Silicon and germanium are both semiconductors with structures coordinating to diamond. Tin has ii mutual allotropes: white (β) tin has a metallic lattice and metallic properties, whereas gray (α) tin has a diamond-like structure and is a semiconductor. The metallic β form is stable above 13.ii°C, and the nonmetallic α class is stable below 13.2°C. Atomic number 82 is the only group 14 element that is metallic in both structure and properties under all conditions.
Video \(\PageIndex{1}\): Time lapse tin pest reaction.
Based on its position in the periodic table, nosotros look silicon to exist amphoteric. In fact, it dissolves in strong aqueous base to produce hydrogen gas and solutions of silicates, but the only aqueous acrid that it reacts with is hydrofluoric acid, presumably due to the formation of the stable SiF6 2− ion. Germanium is more metallic in its behavior than silicon. For example, it dissolves in hot oxidizing acids, such as HNO3 and HiiSOfour, merely in the absence of an oxidant, it does non dissolve in aqueous base. Although can has an even more metallic character than germanium, lead is the just element in the group that behaves purely every bit a metal. Acids do not readily assail it because the solid acquires a thin protective outer layer of a Lead2 + common salt, such as PbSO4.
All grouping xiv dichlorides are known, and their stability increases dramatically as the atomic number of the central cantlet increases. Thus CClii is dichlorocarbene, a highly reactive, short-lived intermediate that can be fabricated in solution but cannot exist isolated in pure class using standard techniques; SiCl2 can exist isolated at very low temperatures, but it decomposes rapidly to a higher place −150°C, and GeCl2 is relatively stable at temperatures beneath xx°C. In contrast, SnCltwo is a polymeric solid that is indefinitely stable at room temperature, whereas PbCl2 is an insoluble crystalline solid with a structure like to that of SnClii.
The stability of the group xiv dichlorides increases dramatically from carbon to pb.
Although the first four elements of group 14 form tetrahalides (MXfour) with all the halogens, only fluorine is able to oxidize lead to the +four oxidation state, giving PbF4. The tetrahalides of silicon and germanium react rapidly with water to give amphoteric oxides (where M is Si or Ge):
\[MX_{4(south,l)} + 2H_2O_{(50)} \rightarrow MO_{2(s)} + 4HX_{(aq)} \characterization{\(\PageIndex{1}\)1}\]
In contrast, the tetrahalides of can and lead react with water to give hydrated metal ions. Because of the stability of its +2 oxidation land, atomic number 82 reacts with oxygen or sulfur to course PbO or PbS, respectively, whereas heating the other grouping 14 elements with excess Otwo or Seight gives the corresponding dioxides or disulfides, respectively. The dioxides of the grouping xiv elements become increasingly basic as we become down the group.
The dioxides of the group 14 elements go increasingly basic down the group.
Because the Si–O bond is even stronger than the C–O bond (~452 kJ/mol versus ~358 kJ/mol), silicon has a strong analogousness for oxygen. The relative strengths of the C–O and Si–O bonds contradict the generalization that bond strengths subtract equally the bonded atoms become larger. This is considering we have thus far assumed that a formal single bond betwixt ii atoms can ever be described in terms of a unmarried pair of shared electrons. In the case of Si–O bonds, however, the presence of relatively low-energy, empty d orbitals on Si and nonbonding electron pairs in the p or spn hybrid orbitals of O results in a partial π bond (Figure \(\PageIndex{three}\)). Due to its partial π double bond grapheme, the Si–O bond is significantly stronger and shorter than would otherwise be expected. A similar interaction with oxygen is also an of import feature of the chemical science of the elements that follow silicon in the third catamenia (P, S, and Cl). Considering the Si–O bond is unusually potent, silicon–oxygen compounds dominate the chemical science of silicon.
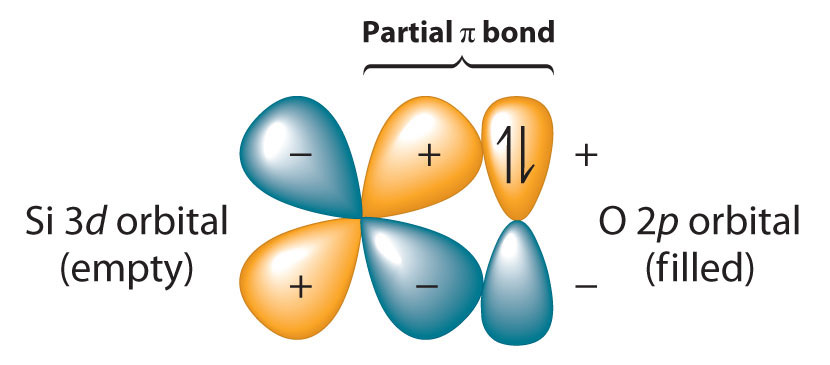
Because silicon–oxygen bonds are unusually strong, silicon–oxygen compounds boss the chemistry of silicon.
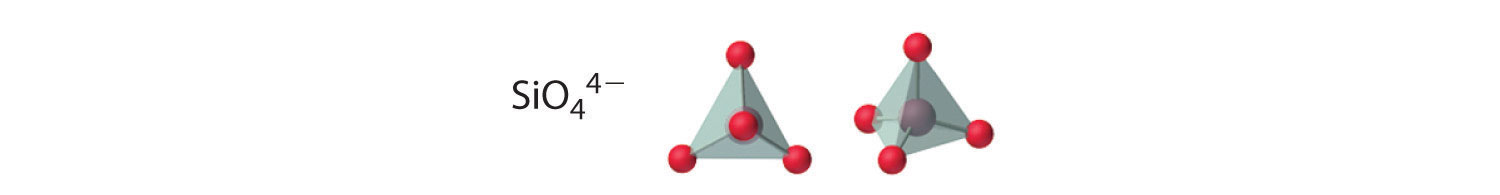
Compounds with anions that incorporate only silicon and oxygen are called silicates, whose basic building block is the SiOiv 4− unit:
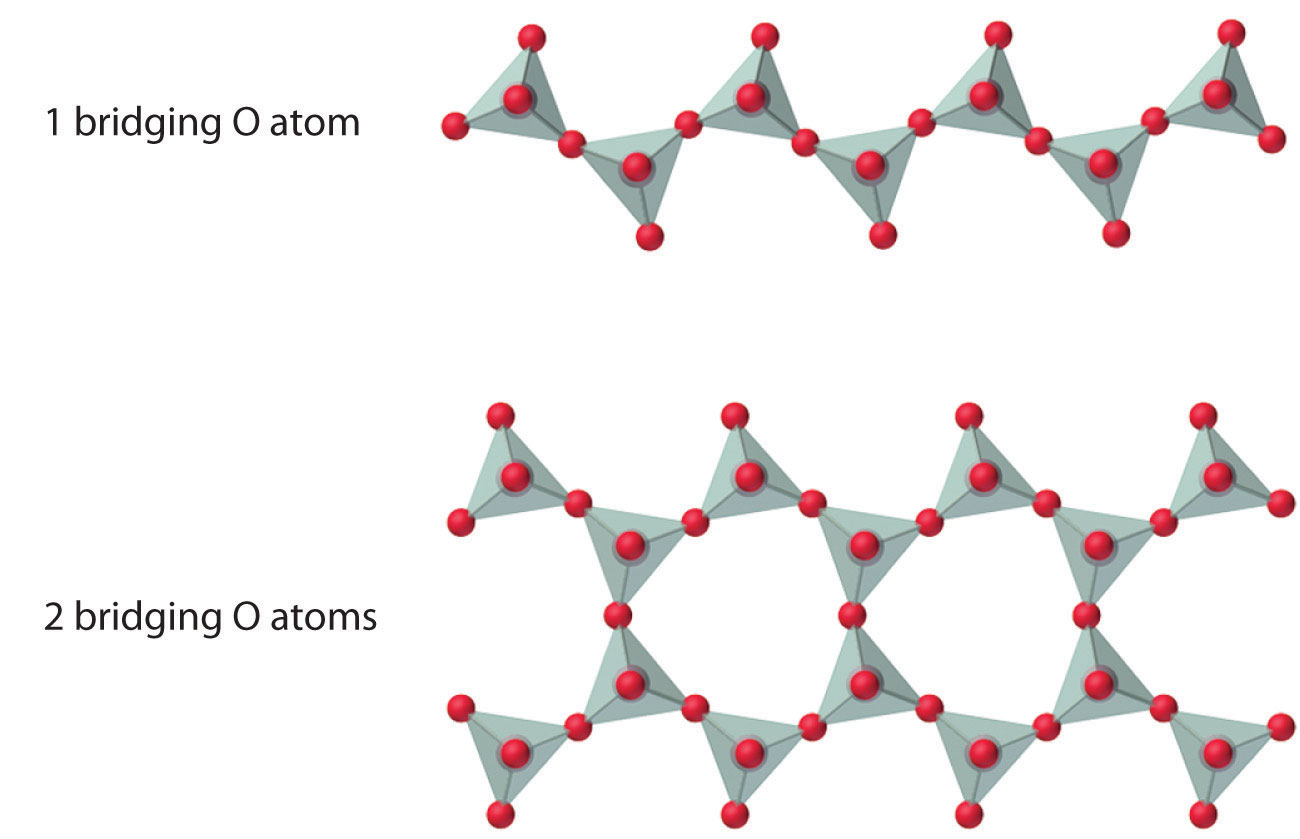
The number of oxygen atoms shared between silicon atoms and the way in which the units are linked vary considerably in different silicates. Converting i of the oxygen atoms from terminal to bridging generates bondage of silicates, while converting two oxygen atoms from terminal to bridging generates double chains. In contrast, converting three or iv oxygens to bridging generates a variety of complex layered and three-dimensional structures, respectively.
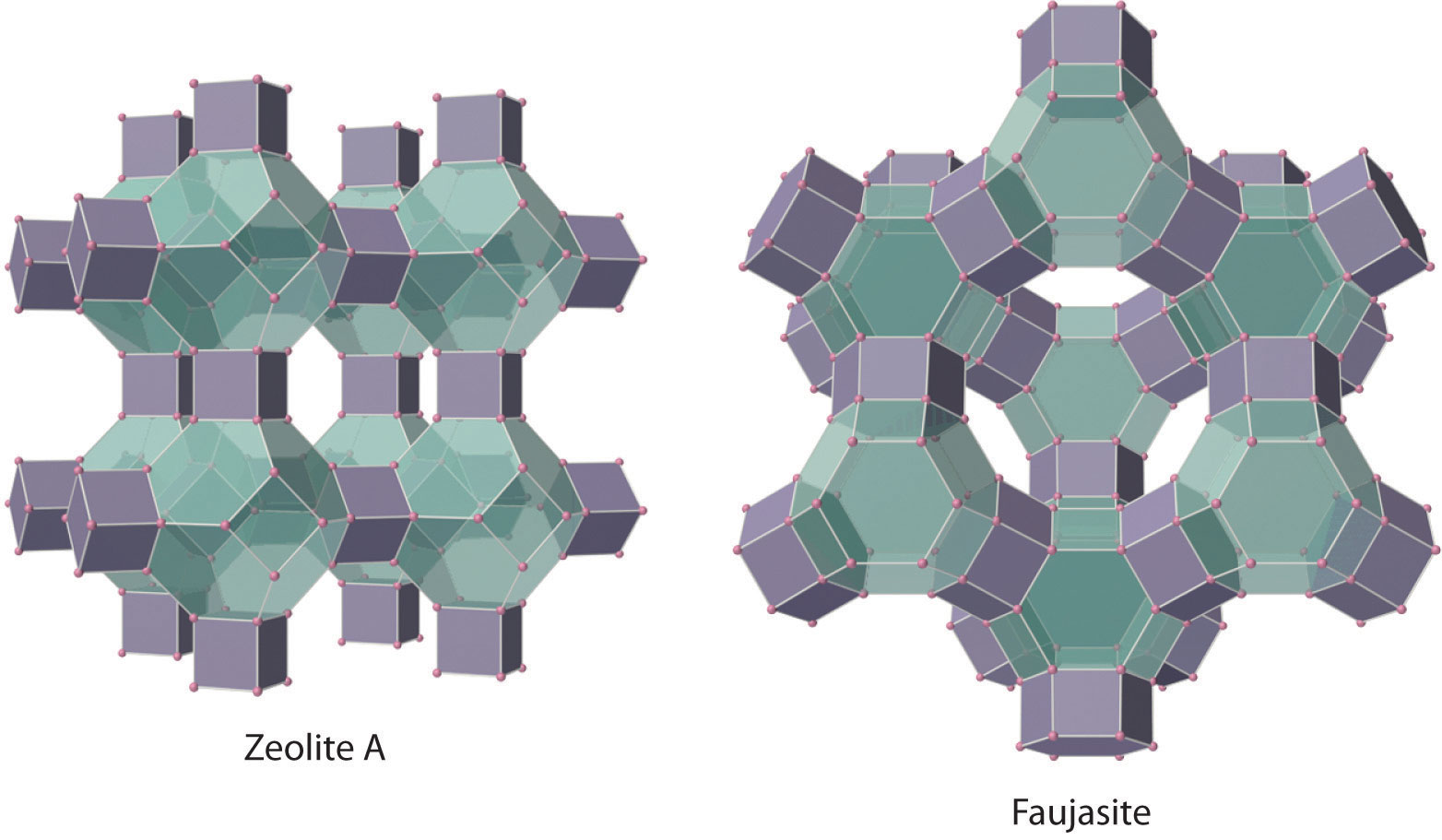
In a large and important course of materials called aluminosilicates, some of the Si atoms are replaced past Al atoms to requite aluminosilicates such as zeolites, whose three-dimensional framework structures have large cavities connected by smaller tunnels (Effigy \(\PageIndex{iv}\)). Because the cations in zeolites are readily exchanged, zeolites are used in laundry detergents as water-softening agents: the more than loosely bound Na+ ions within the zeolite cavities are displaced by the more highly charged Mgtwo + and Ca2 + ions nowadays in hard water, which bind more tightly. Zeolites are also used equally catalysts and for water purification.
Silicon and germanium react with nitrogen at loftier temperature to form nitrides (K3N4):
\[ 3Si_{(l)} + 2N_{2(g)} \rightarrow Si_3N_{4(due south)} \label{\(\PageIndex{1}\)2}\]
Silicon nitride has properties that arrive suitable for high-temperature engineering applications: it is stiff, very hard, and chemically inert, and information technology retains these properties to temperatures of about 1000°C.
Because of the diagonal human relationship between boron and silicon, metal silicides and metal borides exhibit many similarities. Although metallic silicides take structures that are as circuitous every bit those of the metallic borides and carbides, few silicides are structurally similar to the corresponding borides due to the significantly larger size of Si (atomic radius 111 pm versus 87 pm for B). Silicides of active metals, such as Mg2Si, are ionic compounds that comprise the Si4− ion. They react with aqueous acid to form silicon hydrides such as SiH4:
\[Mg_2Si_{(due south)} + 4H^+_{(aq)} \rightarrow 2Mg^{two+}_{(aq)} + SiH_{4(k)} \label{\(\PageIndex{ane}\)iii}\]
Unlike carbon, catenated silicon hydrides become thermodynamically less stable every bit the chain lengthens. Thus straight-concatenation and branched silanes (coordinating to alkanes) are known up to only northward = 10; the germanium analogues (germanes) are known upward to n = 9. In contrast, the only known hydride of tin is SnH4, and information technology slowly decomposes to elemental Sn and H2 at room temperature. The simplest pb hydride (PbH4) is then unstable that chemists are non fifty-fifty certain information technology exists. Considering E=E and E≡E bonds become weaker with increasing atomic number (where Due east is whatever group 14 element), elementary silicon, germanium, and tin analogues of alkenes, alkynes, and aromatic hydrocarbons are either unstable (Si=Si and Ge=Ge) or unknown. Silicon-based life-forms are therefore probable to be found merely in scientific discipline fiction.
The stability of group fourteen hydrides decreases downwards the grouping, and E=East and East≡E bonds become weaker.
The only important organic derivatives of lead are compounds such equally tetraethyllead [(CH3CHtwo)fourPb]. Considering the Pb–C bond is weak, these compounds decompose at relatively depression temperatures to produce alkyl radicals (R·), which can be used to control the rate of combustion reactions. For 60 yr, hundreds of thousands of tons of pb were burned annually in automobile engines, producing a mist of lead oxide particles along the highways that constituted a potentially serious public health trouble. (Example 6 in Section 22.iii examines this problem.) The utilise of catalytic converters reduced the amount of carbon monoxide, nitrogen oxides, and hydrocarbons released into the atmosphere through automobile exhausts, but it did zilch to decrease lead emissions. Because atomic number 82 poisons catalytic converters, however, its use every bit a gasoline condiment has been banned in most of the world.

Compounds that contain Si–C and Si–O bonds are stable and of import. High-molecular-mass polymers called silicones incorporate an (Si–O–)n backbone with organic groups attached to Si (Figure \(\PageIndex{5}\)). The properties of silicones are adamant by the chain length, the type of organic group, and the extent of cantankerous-linking between the chains. Without cross-linking, silicones are waxes or oils, but cantankerous-linking tin produce flexible materials used in sealants, gaskets, car polishes, lubricants, and even elastic materials, such as the plastic substance known as Airheaded Putty.

Case \(\PageIndex{2}\)
For each reaction, explicate why the given products form.
- Atomic number 82(s) + Clii(g) → PbCl2(s)
- MgiiSi(s) + 4H2O(l) → SiH4(g) + 2Mg(OH)ii(southward)
- GeOii(due south) + 4OH−(aq) → GeO4 4−(aq) + 2H2O(50)
Given: balanced chemical equations
Asked for: why the given products form
Strategy:
Classify the type of reaction. Using periodic trends in atomic properties, thermodynamics, and kinetics, explain why the observed reaction products form.
Solution:
- Pb is a metal, and chlorine is a nonmetal that is a potent oxidant. Thus we can expect a redox reaction to occur in which the metal acts as a reductant. Although pb can grade compounds in the +two and +iv oxidation states, Atomic number 824 + is a potent oxidant (the inert-pair effect). Because atomic number 82 prefers the +2 oxidation state and chlorine is a weaker oxidant than fluorine, nosotros wait PbCl2 to be the production.
- This is the reaction of water with a metallic silicide, which formally contains the Si4− ion. Water can act as either an acrid or a base of operations. Because the other compound is a base, we expect an acrid–base reaction to occur in which water acts as an acid. Because MgtwoSi contains Si in its lowest possible oxidation land, still, an oxidation–reduction reaction is also a possibility. Merely h2o is a relatively weak oxidant, so an acid–base reaction is more than likely. The acid (H2O) transfers a proton to the base (Siiv−), which can accept four protons to form SiH4. Proton transfer from water produces the OH− ion, which volition combine with Mgtwo + to requite magnesium hydroxide.
- Nosotros expect germanium dioxide (GeO2) to exist amphoteric considering of the position of germanium in the periodic table. Information technology should dissolve in strong aqueous base to give an anionic species analogous to silicate.
Practice \(\PageIndex{2}\)
Predict the products of the reactions and write a balanced chemical equation for each reaction.
- PbOtwo(due south) \(\xrightarrow{\Delta}\)
- GeCl4(s) + HtwoO(50) →
- Sn(southward) + HCl(aq) →
Answer
- \(\mathrm{PbO_2(s)}\xrightarrow{\Delta}\mathrm{PbO(s)}+\frac{1}{2}\mathrm{O_2(g)}\)
- GeClfour(s) + 2H2O(l) → GeOii(s) + 4HCl(aq)
- Sn(southward) + 2HCl(aq) → Sntwo +(aq) + Htwo(g) + 2Cl−(aq)
Summary
The group 14 elements bear witness the greatest diversity in chemic behavior of any group; covalent bond strengths decease with increasing atomic size, and ionization energies are greater than expected, increasing from C to Pb. The group 14 elements prove the greatest range of chemical behavior of whatsoever group in the periodic tabular array. Because the covalent bond strength decreases with increasing atomic size and greater-than-expected ionization energies due to an increment in Zeff, the stability of the +2 oxidation state increases from carbon to lead. The tendency to form multiple bonds and to catenate decreases as the atomic number increases. The stability of the carbon tetrahalides decreases as the halogen increases in size because of poor orbital overlap and steric crowding. Carbon forms three kinds of carbides with less electronegative elements: ionic carbides, which contain metal cations and C4− (methide) or C2 2− (acetylide) anions; interstitial carbides, which are characterized by covalent metal–carbon interactions and are among the hardest substances known; and covalent carbides, which have three-dimensional covalent network structures that make them extremely hard, high melting, and chemically inert. Consequent with periodic trends, metallic behavior increases down the grouping. Silicon has a tremendous affinity for oxygen because of partial Si–O π bonding. Dioxides of the group xiv elements become increasingly basic down the group and their metallic character increases. Silicates incorporate anions that consist of simply silicon and oxygen. Aluminosilicates are formed past replacing some of the Si atoms in silicates by Al atoms; aluminosilicates with three-dimensional framework structures are called zeolites. Nitrides formed by reacting silicon or germanium with nitrogen are strong, hard, and chemically inert. The hydrides go thermodynamically less stable down the group. Moreover, as atomic size increases, multiple bonds between or to the grouping xiv elements become weaker. Silicones, which contain an Si–O courage and Si–C bonds, are loftier-molecular-mass polymers whose backdrop depend on their compositions.
Contributors and Attributions
-
- Anonymous
What Is The Trend In The Size Of The Band Gap As You Move Down The Column Of The Group 4a Elements?,
Source: https://chem.libretexts.org/Bookshelves/General_Chemistry/Map%3A_Chemistry_(Zumdahl_and_Decoste)/18%3A_The_Representative_Elements/18.06%3A_Group_4A_Elements
Posted by: blackburnupoctin.blogspot.com


0 Response to "What Is The Trend In The Size Of The Band Gap As You Move Down The Column Of The Group 4a Elements?"
Post a Comment